News
Clean scan compels patient to give back to MD Anderson
One day after receiving the most devastating news of his life, Scott Ferguson picked up the phone and called MD Anderson.
It was April 2024, and Scott’s Virginia-based doctor, who had diagnosed him with spindle cell sarcoma the previous July, told him the cancer had metastasized to his lungs.
“My local oncologist said I had only 12 months to live,” Scott recalls. “He told me no treatment would be successful. He told me to give away my money and plan my last trip.”
Scott, who lives in Arlington, Virginia, had already been through a lot. Because of the cancer, his left foot had been amputated in August 2023. He and his doctor had been hopeful that the cancer was gone and would not return.
Yet now that it had, Scott wasn’t ready to stop treatment.
He spoke to a friend who had been an oncology nurse in Washington, D.C.
“She recommended I reach out to MD Anderson,” Scott says. “I called the next morning.”
He was immediately impressed with his patient access representative, Kiara Casteneda.
“She was awesome,” Scott says. “She followed up very quickly on every question, was extremely professional and informative. She helped to assuage my fears with her professionalism and the speed with which I was able to complete the paperwork and scheduling process.”
Within two weeks, Scott was in Houston with Dr. Nakazawa and his team. That marked a new beginning.
Immunotherapy results in no detectable cancer
Michael Nakazawa, M.D., Ph.D., a sarcoma medical oncologist, started Scott on an intense chemotherapy regimen.
“It was hard,” Scott admits. “I vomited for almost a year.”
But the tumors shrank. And once chemo was complete, given the next-generation sequencing results of his tumor, Scott began immunotherapy with pembrolizumab. He still takes the drug, which helps his immune system fight cancer cells, and the results have been impressive.
In July 2025, Ferguson had his first clean CT scan. There was no detectable cancer.
“MD Anderson saved my life,” Scott says. “I feel such a debt of gratitude to Dr. Nakazawa and Juliana Thomas, his advanced practice provider, and Courtney Liberty, his nurse, who recently took a new role in another department. Not only have they been great resources, but they are all truly extraordinary people. Their unwavering support and genuine kindness made the darkest moments of my journey feel hopeful.”
In addition to constant nausea during his initial chemotherapy, Scott experienced challenging symptoms during his cancer treatment, including insomnia, diarrhea and uncontrollable hiccups.
“Dr. Nakazawa’s team always responded promptly with solutions and mitigations that helped,” he says. “Their advice was always technically excellent and compassionate. What made the biggest difference was how they treated me as a whole person, not just a patient — they took the time to truly understand what I was going through and made me feel valued and heard.”
Putting MD Anderson in his will
This happy shift in Scott’s health prompted another shift – this time in his estate plans.
“My wife said, we have to change our will and include MD Anderson,” Scott recalls. He knew she was right based on the treatment he’d received here.
“We don’t have any children,” he says. “We have a nephew who will inherit some of our money, but we are splitting the other half between MD Anderson and a few charities.”
They hope their legacy gift will advance MD Anderson’s mission to end cancer by helping other patients have the same outstanding level of care.
Related: Learn about adding a gift to MD Anderson to your will.
Cancer care and expertise only possible at MD Anderson
Scott still sees his local oncologist in Virginia, who administers his MD Anderson-directed immunotherapy infusion every three weeks.
Every nine weeks, Scott flies to Houston to visit his team at MD Anderson and get lab work, a CT scan, an infusion and a consultation.
Each time he comes to Houston, he says, the news he receives about his health gets better. That’s because of MD Anderson’s standard of care and commitment to excellence.
“When I was working, my teams developed service delivery and quality assurance standards that enabled us to continually improve our service delivery processes over time,” says Scott, who retired at 56 after spending most of his career in the private sector, contracting with federal civilian agencies who outsourced IT, software development or infrastructure operations to his companies.
“When I came to MD Anderson, I realized that every time someone does a scan or takes my blood, they do it the exact same way. That means someone established standard processes, has audited these actions and improved them over time. A standard of excellence is always being followed, and that is one of the many reasons MD Anderson is such a special place.”
To anyone facing a cancer diagnosis, Scott would urge them to seek treatment at MD Anderson.
“Go to the experts,” he says. “Go to the place where they’re creating the science and doing more clinical trials than anyone else. Go to where you’ll find the highest standard of care.”
Request an appointment at MD Anderson online or call 1-877-632-6789.
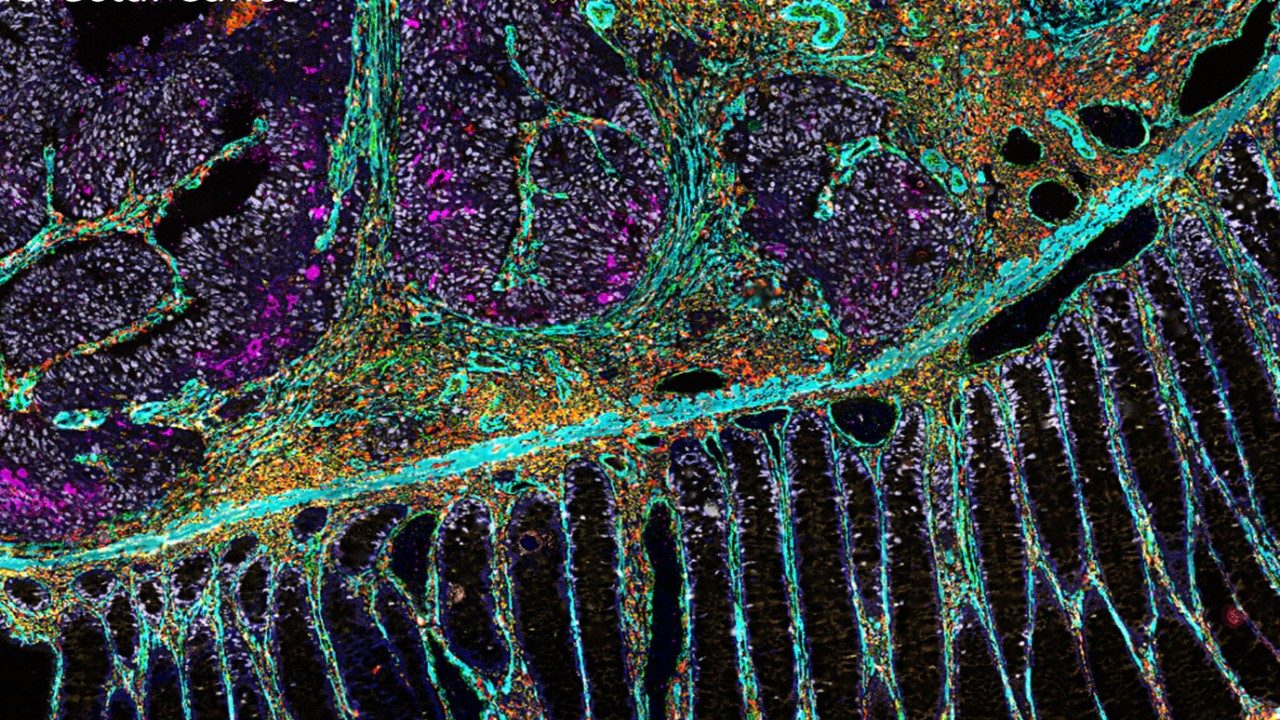
How beautiful images can advance immunotherapy
They say a picture is worth 1,000 words.
In the case of MD Anderson’s immunotherapy platform, part of the James P. Allison Institute, a picture generated by spatial omics technology provides a wealth of information to bring immunotherapy to more patients.
Immune checkpoint inhibitors can treat a variety of cancers, but patients respond differently to these combination therapies based on their unique tumor microenvironment, which is made up of many cell types, including:
- tumor cells
- immune cells
- fibroblasts
- blood vessels
- other cellular components
The immunotherapy platform uses breakthrough imaging and bioinformatics tools to paint a clear picture of the tumor microenvironment before and after immunotherapy treatment. This allows clinicians and researchers to see which cell types are present within the tumor microenvironment and how the cell types change after treatment is given. These data help researchers develop more personalized strategies to target specific cell subsets and improve responses for future patients.
We spoke with Sonali Jindal, M.D., associate director of the immunotherapy platform, to understand how these beautiful images are advancing immunotherapy treatments.
What is spatial omics, and why does it matter?
Spatial omics refers to advanced molecular techniques that analyze biological molecules within their exact location in tissue samples, creating snapshots of the tumor microenvironment. This allows researchers to see the distribution of genes and proteins being expressed, different cell-states and cell-to-cell interactions at the time the sample was collected.
The tumor microenvironment is an ecosystem that includes tumor and immune cells, blood vessels, fibroblasts and signaling proteins in and around a tumor, many of which have their own unique functions and interactions with each other, creating unique cell neighborhoods. Researchers have learned that these neighborhoods can affect whether immune cells are able to recognize and attack cancer cells and influence a tumor’s resistance to treatment.
How does the immunotherapy platform utilize spatial omics?
The immunotherapy platform aims to evaluate immune responses in patients in order to understand which specific therapies or combination therapies will need to be given so that all patients can benefit from immunotherapy. Our platform collects samples, including tumor and blood samples, for immune monitoring from patients enrolled in immunotherapy studies at MD Anderson. More than 5,000 patients have enrolled from over 100 clinical studies across various cancer types.
The samples are carefully tracked and analyzed by a collaborative network of clinicians, physician-scientists and bioinformaticians who can provide real-time monitoring of patients enrolled in clinical trials. Spatial omics is a critical part of that analysis.
How do you create these beautiful images?
We use imaging to gain detailed information about the tumor microenvironment from these samples. Each unique type of cell has certain targets that can be tagged with a marker, usually an antibody or probe. These targets are stained using fluorescent dyes.
It works similar to “paint by number” instructions: each color corresponds to a specific target being studied. Advanced imaging tools then capture these colored sections, mapping out where each cell type or molecule is located.
For the last 50 to 60 years, it was only possible to color one or two markers on a given sample, which provided very basic information on a small scale. Even when researchers added up to nine color markers at a time, it still limited the amount of information that could be generated, given the complex environment.
Fortunately, we have new technology that significantly improves our depth of analysis. For example, CODEX (CO-Detection by indEXing) technology allows simultaneous image staining of dozens of proteins, cells and other targets in a single tumor sample. This technology also attaches a unique barcode to each antibody in order to track and quantify each target.
We want to make sure that we’re able to accurately detect the biologically relevant markers within the tumor microenvironment so that we can integrate all of the data to understand the cell-to-cell interactions and the interactions of specific markers. The data from these studies provide information about why some patients respond – or don’t respond – to treatment and which specific markers need to be targeted with new treatments so that immunotherapy can lead to clinical benefit for more patients.
How does the platform use these images to guide patient care?
The immunotherapy platform provides detailed datasets regarding the tumor microenvironment across thousands of patients, enabling researchers to focus in on specific cell subsets or molecular targets that may be important for the development of new therapies or the selection of specific patient groups for immunotherapy treatments.
We at the immunotherapy platform are very involved in studying each sample, with analyses of hundreds to thousands of molecular markers using various “omic” assays, to determine how cells are responding to treatments, and defining the specific cellular and biologic pathways that drive immune response to eliminate tumor cells.
This allows researchers to generate road maps of the different cellular interactions and neighborhoods involved in various types of cancer response or resistance to immunotherapy.
Generating and analyzing these comprehensive images is no easy task, but it is one that I consider paramount to my role. It is a big privilege for all of us to be able to do this at MD Anderson’s truly collaborative environment, where clinicians and researchers work together so closely. These beautiful pictures are a way to show the impact of immunotherapy so patients can see and understand what can be done for them. It’s all about helping patients. That is what the platform strives for.
Learn about research careers at MD Anderson.
Stage IV bladder cancer caregiver: Why I’ll support MD Anderson until the day I die
When my late husband, Ronnie, was diagnosed with stage IV bladder cancer in 2015, we both knew that our days together were numbered. The disease was not only in his bladder’s muscle wall, but also in the fatty tissue around it and multiple bones. So, his prognosis was pretty grim. Ronnie had maybe three to six months left to live.
We desperately hoped to have more time. So, we turned to MD Anderson. I’ll be forever grateful that we did. Because an immunotherapy clinical trial there suggested by genitourinary cancer specialist Dr. Jianjun Gao gave us 10 extra years together.
How we filled our ‘bonus’ years
Ronnie and I don’t have any children. But we both enjoyed traveling very much. Having a particular type of travel trailer was his lifelong dream. So, after the first few scans revealed the extent of his disease, Ronnie said, “Do you think maybe I could get one?” Of course, I said, “Yes.”
These last 10 years traveling together have been the highlight of my life. We took so many marvelous trips. We went all the way up to Prince Edward Island and Nova Scotia one time, to a good friend’s ranch in Montana another, and to an International Airstream Rally in Rock Spring, Wyoming, too.
Everywhere we went, we bought stickers to commemorate our travels. We attached them to a plexiglass kickplate on our trailer’s door. After Ronnie passed in May, I decided to sell the trailer. But I took that kickplate to a local craft store first to have the stickers removed and framed so I could remember all our happy travels together. I never want to forget them.
4 questions with immunology researcher Susan Bullman
Susan Bullman, Ph.D., has studied bacteria and how it contributes to cancer for decades. Her research led her from her home in Ireland and eventually to MD Anderson and the James P. Allison Institute, where she serves as an associate member.
We asked Bullman four questions about her research and her career. Here’s what she had to say.
How did you get your start as a cancer researcher?
During my graduate studies in Ireland, I focused on understanding how bacteria contribute to gastrointestinal diseases, though I wasn’t working on cancer at the time. Around that period, the Human Microbiome Project was uncovering groundbreaking insights into how microbes influence human health and disease, including cancer. I was fascinated by the idea that these tiny organisms could profoundly impact complex diseases like cancer, shaping everything from its progression to treatment outcomes. I knew this was where I needed to direct my efforts — to explore the critical role these microbes play in cancer biology.
To pursue this, I realized I needed to step out of my comfort zone. I made the life-changing decision to move across the Atlantic. I worked at a few different U.S. institutes focused on cancer research and strengthened my understanding of the complex relationship between microbes and cancer. Eventually, I shaped the central focus of my research program: determining how the microbes within us influence cancer biology and uncovering new possibilities to improve patient outcomes.
You’ve been studying the human microbiota and cancer for over a decade. What most excites you about this area?
What excites me most is the incredible interplay between the human and microbial components of our bodies. For every human cell we have, there are approximately 1.3 microbial cells, and for every human gene, we have 150 microbial genes. This means our bodies are a remarkable union of human and microbial systems, working together in ways we are only beginning to understand. In microbiome science, we focus on how the microbial component impacts the human component — especially because the microbial component is malleable. We can modify and manipulate it, giving us a unique opportunity to influence health and disease.
In cancer biology, microbes are far from passive bystanders. They actively shape the tumor microenvironment, influence immune responses and even affect how a patient responds to therapy. If we can determine how these microbes contribute to cancer, it opens the door to entirely new therapeutic avenues. There’s immense potential to harness this knowledge to develop innovative, personalized diagnostics and therapies, and that’s what continues to inspire and push forward my work in this field.
What’s next for your lab?
Our lab is focused on uncovering how specific microbes infiltrate human tumors and alter the behavior of cancer. Our previous work has shown that these microbes not only infiltrate tumors but also interact with immune cells and cancer cells within the tumor microenvironment, contributing to poorer outcomes for patients. A major focus of our research is understanding why these microbes are associated with a worse prognosis and how they impact the way patients respond to cancer treatments.
To tackle these questions, we are adapting and applying advanced technologies like single-cell spatial transcriptomics and proteomics. These tools allow us to precisely map microbes within human tumors and investigate the mechanisms by which they help cancer grow and survive. By identifying microbial biomarkers and determining how these microbes interact with the tumor microenvironment, we aim to intercept these harmful interactions and develop new therapeutic strategies. Another key focus is on creating narrow-spectrum antimicrobials targeting Fusobacterium nucleatum, a microbe that contributes to colorectal cancer, with the goal of improving patient responses to current cancer treatments and reducing the risk for cancer relapse or metastases.
You joined MD Anderson last year. What about MD Anderson excites you most?
What excites me most is MD Anderson’s highly collaborative environment. There is a clear focus on asking: How can this work? How can these discoveries reach patients? The James P. Allison Institute exemplifies this approach, seamlessly transitioning groundbreaking discoveries from the lab to the clinic. Basic and translational researchers collaborate closely with clinical trialists, medicinal chemists, oncologists, and surgeons to maximize the potential for research to directly impact patients.
MD Anderson offers a truly unique environment to ensure that my research has a real-world impact. I want my work to be meaningful — I don’t want it to remain confined to the research lab. I want it to reach patients, where it can truly make a difference. MD Anderson is unparalleled in its ability to integrate research findings into the clinical setting, bridging the gap between discovery and patient care.
Having worked at major cancer centers across the U.S., I can confidently say that MD Anderson is uniquely positioned to transform research into action. Being part of this institution gives me the opportunity to move my work beyond the lab and into the clinic, where it can hopefully help patients with cancer — a mission that is deeply motivating.
More News
-
Cancerwise
Next-generation sequencing and immunotherapy give bladder cancer patient hope
-
Cancerwise
Researcher studies tumor cell interaction to improve immunotherapy
-
News Release
Allison Institute scientific symposium features spatial biology breakthroughs, $5 million gift to support further efforts
-
News Release
Allison Institute and Institute for Data Science in Oncology blend spatial biology efforts by appointing new member
-
News Release
Targeting myeloid cell protein overcomes pancreatic cancer immunotherapy resistance
-
News Release
Immunosuppressive therapy after checkpoint inhibitors can reduce side effects without compromising antitumor activity
-
News Release
Allison Institute announces appointment of two immunobiology experts as associate members
-
News Release
MD Anderson Research Highlights for April 12, 2024
-
News Release
MD Anderson's Padmanee Sharma elected Fellow of the AACR Academy
-
News Release
Is AI the cancer-fighting tool we've been waiting for? Opens a new window
-
News Release
Allison Institute hosts inaugural scientific symposium
-
News Release
MD Anderson Research Highlights for September 21, 2023
-
News Release
Allison Institute announces appointment of inaugural members
-
BBC Radio 4
Moths and miracles Opens a new window
-
News Release
A Conversation With a Living Legend® San Antonio honors Nobel Laureate, raises funds for James P. Allison Institute
-
News Release
Andrew Sabin Family Foundation doubles down on commitment to end cancer with $10M gift
-
News Release
Timken Foundation advances mission to end cancer with $5 million gift to James P. Allison Institute
-
News Release
IBC Bank's campaign to end cancer tops $2.2 million
-
News Release
Allison Institute establishes internal advisory council of MD Anderson experts
-
News Release
Allison Institute announces formation of scientific advisory board
-
CNN
The future of cancer research Opens a new window
-
Forbes
If you've ever had a great idea snubbed, this Nobel Laureate wants you to know you're not alone Opens a new window
-
Cancerwise
What is the future of immunotherapy?
-
News Release
MD Anderson launches James P. Allison Institute to usher in new era for immunotherapy