News and Media
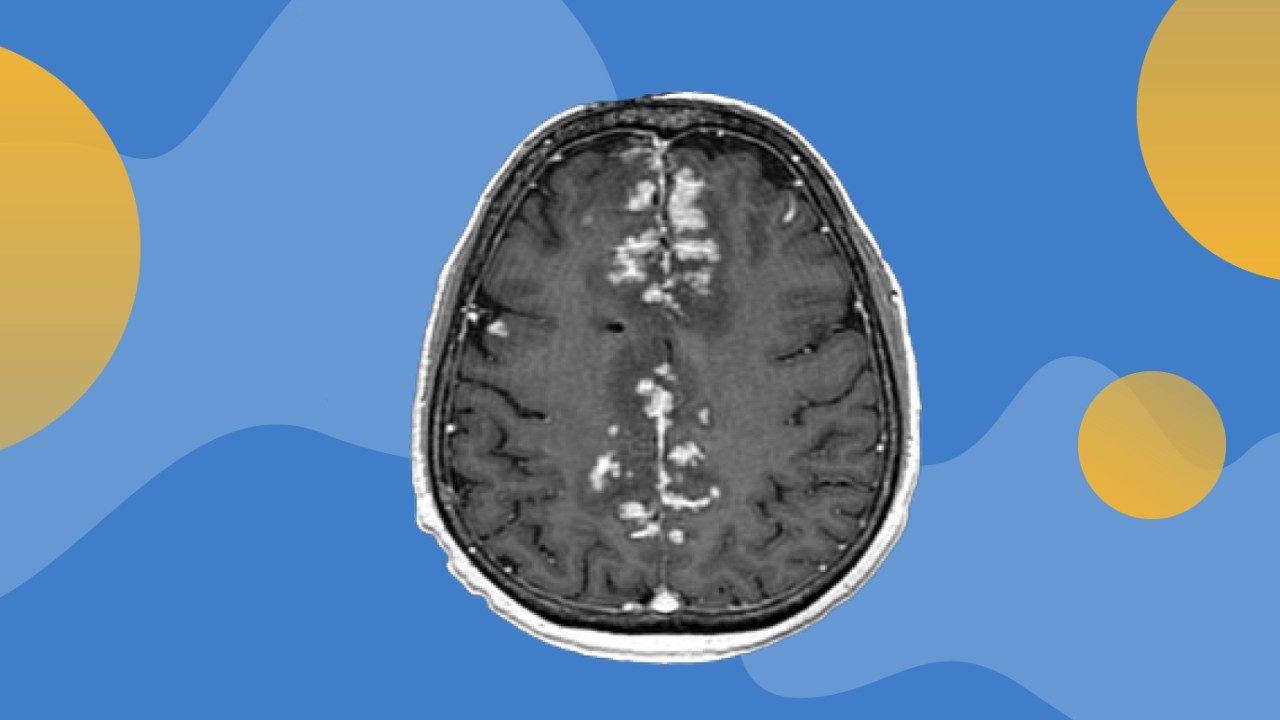
Novel immunotherapy delivery approach safe and beneficial for some melanoma patients with leptomeningeal disease
A novel approach to administer intrathecal (IT) immunotherapy (directly into the spinal fluid) and intravenous (IV) immunotherapy was safe and improved survival in a subset of patients with leptomeningeal disease (LMD) from metastatic melanoma, according to interim analyses of a Phase I/Ib trial led by researchers at The University of Texas MD Anderson Cancer Center.
The study, published today in Nature Medicine, represents the first-in-human trial of concurrent IT and IV nivolumab (anti-PD-1) in melanoma patients with LMD. Across 25 patients, the median overall survival (OS) was 4.9 months, with OS rates of 44% at 26 weeks and 26% at 52 weeks. Four patients survived to 74, 115, 136 and 143 weeks after their first IT dose, which is significantly longer than expected.
“This represents a major path forward for our patients, as there is a crucial unmet clinical need for better treatments for patients with LMD,” said corresponding author Isabella Glitza Oliva, M.D., Ph.D., associate professor of Melanoma Medical Oncology. “We are encouraged by these preliminary results for a disease that has been notoriously difficult to study due to its highly aggressive nature. This approach is safe, and we’re seeing a small subset of our patients who have had outstanding results, so we hope to learn from each and every one of them.”
Leptomeningeal disease is a complication of cancer that occurs when cancer cells from primary tumors migrate into the cerebrospinal fluid (CSF) and leptomeninges, the outer lining of the brain and spinal cord. These cells can quickly spread throughout the CSF and cause a wide variety of neurological symptoms. Roughly 10% of patients with stage IV melanoma will be diagnosed with LMD, which also commonly derives from metastatic lung cancer and breast cancer.
There is no cure for LMD, but treatments such as targeted therapy and immunotherapy may improve quality of life. While immune checkpoint inhibitors are beneficial in patients with metastatic melanoma, little is known about their potential use for treating LMD.
Intrathecal administration has been studied in other settings. A previous proof-of-concept study by Glitza Oliva and colleagues at MD Anderson demonstrated that IT administration of interleukin-2 in patients with LMD had encouraging results but was associated with serious side effects. This new study showed that injecting nivolumab directly into the spinal fluid increases its concentration within the CSF, since these antibodies cannot otherwise easily penetrate the blood-brain barrier
The current trial enrolled 25 patients with a median age of 43 and all but two had received prior systemic therapy, including immune checkpoint inhibitors (84%), BRAF/MEK inhibitors (64%) and chemotherapy (12%). The dose-expansion cohort evaluated four doses of IT nivolumab concurrent with a flat dose of IV nivolumab.
The drug was well tolerated at the highest IT nivolumab dose, with only mild grade 1 or 2 side effects and no dose-limiting toxicities. The most common treatment-related adverse events were nausea, dizziness and vomiting.
“Until recently there have been limited resources to develop clinical trials in this space, but we owe it to patients with very challenging diseases to push the unknown and to advocate for them when they don’t have many options,” Glitza Oliva said. “We are optimistic that these results, along with further clinical trials, will lead us to a better understanding of LMD and, ultimately, more effective ways of helping our patients.”
Recently, the study completed enrollment for the dose-expansion cohort; analysis is underway to provide an opportunity for further insights. Ongoing research will seek to identify biomarkers that may predict patients most likely to benefit from this treatment approach.
This work was supported by Bristol-Myers Squibb Foundation, Inc. Additional funding was provided by the National Cancer Institute (1P50CA221703-01A1), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and MD Anderson’s Moon Shots Program®. A full list of collaborating authors and their disclosures can be found with the full paper here.

Neoadjuvant immunotherapy with relatlimab and nivolumab is safe and effective in stage III melanoma
Giving the combination of immune checkpoint inhibitors relatlimab and nivolumab to patients with stage III melanoma before surgery was safe and completely cleared all viable tumor in 57% of patients in a Phase II study, researchers from The University of Texas MD Anderson Cancer Center reported in Nature today.
In addition to meeting the primary endpoint of pathologic complete response (pCR), the overall pathologic response rate (up to 50% of viable tumor left at the time of surgery) was 70%. No patients had grade 3 or 4 immune-related adverse events (IRAEs) in the neoadjuvant (pre-surgery) setting or confirmed toxicity-related surgical delays.
“With clinical stage III melanoma, the risk that the cancer comes back after surgery can be as high as 50%. One of the goals of neoadjuvant immunotherapy is to reduce the chance of recurrence by evaluating treatments in earlier stage, operable disease that have been successful for stage IV melanoma,” said lead/corresponding author Rodabe Amaria, M.D., associate professor of Melanoma Medical Oncology. “Our findings support the combination of relatlimab and nivolumab as a safe and effective treatment option in the neoadjuvant setting for stage III melanoma.”
Relatlimab is a novel immune checkpoint inhibitor that blocks LAG-3, which is found on the surface of T cells and often is upregulated in melanoma. Nivolumab is a PD-1 inhibitor. The Food and Drug Administration approved the same combination for stage IV melanoma in March 2022 based on results of the RELATIVITY-047 study, reported by MD Anderson in the New England Journal of Medicine in January 2022.
The data also build on recent encouraging results for single-agent neoadjuvant immunotherapy presented by MD Anderson at the European Society for Medical Oncology (ESMO) Congress 2022 for stage III-IV melanoma and stage II–IV cutaneous squamous cell carcinoma.
The study enrolled 30 patients at MD Anderson and Memorial Sloan Kettering Cancer Center to receive two doses of neoadjuvant relatlimab and nivolumab, followed by surgery and 10 doses of the same combination in the adjuvant setting. One patient developed a brain metastasis during neoadjuvant therapy and did not proceed to surgery. The median age of patients was 60, and 63% of patients were male.
After a median of 24.4 months follow-up in the 29 patients who underwent surgery, the rate of recurrence-free survival (RFS) was 97% at one year and 82% at two years. RFS rates were highest in patients with a pCR: 100% at one year and 91% at two years, compared to 92% and 69% in patients without a pCR. The one- and two-year overall survival rates for all patients were 93% and 88%, respectively.
No grade 3 or 4 IRAEs occurred during the eight weeks of neoadjuvant treatment. One patient’s surgery was delayed due to asymptomatic myocarditis, which was determined to be unrelated to treatment. During adjuvant therapy, 26% of patients developed grade 3 or 4 IRAEs, with secondary adrenal insufficiency and elevated liver enzymes being two of the most common side effects.
The results are favorable in relation to two prior study arms, which assessed nivolumab either alone or in combination with the CTLA-4 checkpoint inhibitor ipilimumab. Those results, reported by Amaria and colleagues in Nature Medicine in 2018, showed a pCR rate of 45%, with 73% of patients experiencing grade 3 side effects in the combination arm. The nivolumab monotherapy arm achieved a pCR rate of 25%, with 8% of patients experiencing grade 3 side effects. The high toxicity rate led to early closure of the previous study.
“We’re very pleased to see that the combination of relatlimab and nivolumab balanced safety and efficacy, and it did not result in any delays to surgery,” Amaria said. “We want to provide patients with a treatment option that will help reduce the risk of their cancer returning after surgery. Our data complement the RELATIVITY-047 study results and provide further evidence to support the use of this combination in melanoma.”
Translational studies of blood and tissue samples revealed that a greater presence of immune cells at baseline and decreases in M2 macrophages during treatment were associated with pathological response. Pathologic response was not correlated with LAG-3 and PD-1 levels in baseline tumor samples. Further follow-up is needed to determine the impact on overall survival and biomarkers of response.
The study was funded by Bristol Myers Squibb (BMS). Amaria has worked in a consulting/advisory role for and received research/grant support from BMS. MD Anderson authorship included co-lead author Elizabeth Burton and co-senior authors Padmanee Sharma, M.D., Ph.D., Jennifer Wargo, M.D., and Hussein Tawbi, M.D., Ph.D. A full list of co-authors and disclosures can be found in the paper.

Targeting interleukin-6 could help relieve immunotherapy side effects
Researchers at The University of Texas MD Anderson Cancer Center have identified a novel strategy to reduce immune-related adverse events from immunotherapy treatment by targeting the cytokine interleukin-6 (IL-6). The study, published today in Cancer Cell, establishes a proof of concept for combining immune checkpoint blockade with cytokine blockers to selectively inhibit inflammatory autoimmune responses.
While combination immunotherapy with anti-PD-1 and anti-CTLA-4 agents has revolutionized treatment for multiple cancer types, it also has high toxicity rates, which can affect quality of life and lead to treatment discontinuation. Often, patients whose cancers respond to combination immunotherapy also experience high-grade side effects. Immune-related enterocolitis (irEC), an inflammatory bowel condition, is the most common serious complication.
“We need to overcome immune toxicity, first and foremost, to support patients and reduce their symptom burden,” said senior author Adi Diab, M.D., associate professor of Melanoma Medical Oncology. “Secondly, we know that there are multiple, non-overlapping mechanisms of resistance in the tumor microenvironment. In order to build an effective multi-agent immunotherapy regimen, we have to overcome the barrier of immune-related toxicity so that patients can continue receiving the optimum treatment.”
The translational study analyzed patient tissue, preclinical models and retrospective data to determine how the IL-6 T-helper 17-cell (Th17) pathway contributes to toxicity and can be inhibited to separate the inflammatory autoimmune response from the antitumor immune response.
Preclinical studies reveal immunobiology of immune-related adverse events
IL-6 has been associated with immunotherapy resistance in preclinical models, but the mechanism was not well understood. IL-6 also is associated with several autoimmune diseases, and IL-6 blockers are approved to treat rheumatologic disorders and other autoimmune conditions.
Comprehensive immune profiling of matched samples of irEC tissue and normal tissue from patients treated with immune checkpoint blockade (12 patients in the observation cohort and 11 in the validation cohort) revealed distinct immune signatures in the inflamed tissue (where IL-6 and Th17 were upregulated) compared to normal tissue. Furthermore, the IL-6 gene signature was upregulated in those whose tumors did not respond to immunotherapy, but the increased levels were not seen in responders.
Based on this observation, the researchers then used several preclinical models to evaluate the effect of an IL-6 blockade on autoimmunity and on response to anti-CTLA-4 therapy. The combination of an IL-6 blocker with immune checkpoint inhibitor decreased experimental autoimmune encephalomyelitis (EAE) symptoms and improved tumor control, indicating that the combination could suppress inflammatory response and potentially enhance antitumor immunity.
Observational cohort validates IL-6 strategy, prospective clinical trial in progress
To validate the findings, the researchers performed a retrospective analysis of 31 patients with melanoma who were treated with immune checkpoint blockade between January 2004 and March 2021 and also received an IL-6 blocker to treat inflammatory arthritis and other immune-related adverse events. Patients in the cohort received IL-6 blockade a median of 3.7 months after beginning to experience side effects, and the researchers noted a 74% improvement in symptoms after a median of two months on IL-6 blockade therapy.
Of the 26 patients with evaluable tumor response before (or early in) IL-6 blockade therapy and at follow-up, the best overall response rate to immune checkpoint blockade was 57.7% before IL-6 blockade initiation and 65.4% after therapy. These clinical results supported the preclinical findings, which determined that targeting IL-6 can alleviate immune-related adverse events without compromising the efficacy of immunotherapy.
“Cytokine blockers have been well established to block autoimmunity. The novelty of this study is bringing cytokine targeting to tumor immunity and demonstrating that autoimmunity and antitumor immunity are not necessarily overlapping immune responses but can be decoupled at the cytokine level,” Diab said. “IL-6 is only one cytokine, but this work offers proof of principle for taking the science to the next level by targeting multiple cytokines in a multi-layered approach.”
Based on these results, Diab is leading an investigator-initiated Phase II prospective clinical trial (NCT04940299) to assess the safety and efficacy of IL-6 blockade in combination with anti-PD-1 and anti-CTLA-4 therapy in several different cancer types.
This study was supported by Wilkes Family Cancer Autoimmune Research Fund, with additional research support from the American Society of Clinical Oncology/Conquer Cancer Foundation, National Institutes of Health/National Cancer Institute (P30 CA016672, P50CA221703) and National Institute of Allergy and Infectious Diseases (K01AI163412). Diab reports research support and advisory board fees from Bristol Myers Squibb. A full list of co-authors and disclosures is available in the paper.

MD Anderson receives nearly $19.4 million in CPRIT funding
The University of Texas MD Anderson Cancer Center today was awarded 15 grants totaling $19.38 million from the Cancer Prevention and Research Institute of Texas (CPRIT) in support of cancer research projects across the institution.
“We are grateful for CPRIT’s invaluable support of MD Anderson’s mission to end cancer,” said Peter Pisters, M.D., president of MD Anderson. “The funds awarded today will strengthen the ability of our world-leading team of dedicated scientists and clinicians to test new hypotheses, drive scientific discoveries and generate breakthroughs that can improve the lives of cancer patients.”
Since its inception, CPRIT has awarded more than $3 billion in grants for cancer research. MD Anderson investigators have received $587 million, approximately 20% of the total awards. Programs supported by CPRIT funding have brought more than 288 distinguished cancer researchers to Texas, advanced the knowledge base for cancer treatment throughout the state and provided more than 8.2 million cancer prevention and early detection services reaching all 254 counties in Texas.
CPRIT awards to MD Anderson include:
Individual Investigator Research Awards:
- Neuronal alterations in the midbrain dopaminergic system function: a novel mechanism of radiation-induced cognitive dysfunction (Die Zhang, Ph.D., Radiation Oncology) - $1,399,730
- Geospatial approaches to melanoma early detection – The GAMED Project (Kelly Nelson, M.D., Dermatology) - $1,998,196
- A novel therapeutic strategy targeting pancreatic cancer (Wantong Yao, M.D., Ph.D., Translational Molecular Pathology) - $1,049,854
- Studying and therapeutically targeting ferroptosis liability in BRCA1 deficient cancer (Boyi Gan, Ph.D., Experimental Radiation Oncology) - $1,050,000
- Targeting the Mlc1-GlialCAM protein complex in invasive glioma cells (Joseph McCarty, Ph.D., Neurosurgery) - $1,050,000
- Blocking DNA damage response induction of “don’t eat me” signals converts local radiotherapy into systemic immunotherapy (Michael Curran, Ph.D., Immunology) - $1,049,905
- Normalizing membrane homeostasis in microglia/macrophages of pediatric high-grade gliomas (Jian Hu, Ph.D., Cancer Biology) - $1,400,000
- Overcoming therapy resistance by integrated computational modeling of the bone metastatic niche in prostate and renal cancers (Eleonora Dondossola, Ph.D., Genitourinary Medical Oncology) - $1,025,623
- Integrative modeling of spatially resolved multi-omics data to identify bladder cancer mucosal field effects (Peng Wei, Ph.D., Biostatistics) - $1,199,994
- Multi-component interventions to improve uptake and adherence to lung cancer screening (Robert Volk, Ph.D., Health Services Research) - $1,988,211
- Enhancing immune responses in pancreatic cancer by stromal inhibition of HIF2 (Cullen Taniguchi, M.D., Ph.D., GI Radiation Oncology) - $1,049,997
- A novel combination therapeutic approach to revitalizing immunotherapy for bone metastatic prostate cancer (Sue-Hwa Lin, Ph.D., Translational Molecular Pathology) - $1,050,000
- Targeting distinct metabolic vulnerabilities of aggressive renal cell carcinoma variants (Niki Zacharias Millward, Ph.D., Urology) - $1,019,997
- Mapping the geospatial tumor clonal architecture to restore immune recognition in pancreatic cancer (Andrea Viale, M.D., Genomic Medicine) - $1,049,985
Scholar Recruitment Awards:
- Recruitment of one first-time, tenure-track faculty member - $2,000,000

High-fiber diet associated with improved progression-free survival and response to immunotherapy in melanoma patients
Patients with melanoma who reported eating more fiber-rich foods when they began immunotherapy treatment survived longer without cancer growth than patients with insufficient dietary fiber intake, according to new research from The University of Texas MD Anderson Cancer Center published today in Science. The benefit was most noticeable in patients who did not take commercially available probiotic supplements. Parallel pre-clinical studies supported the observational findings.
“Research from our team and others has shown that gut microbes impact response to immunotherapy treatment, but the role of diet and probiotic supplements has not been well studied,” said co-senior author Jennifer Wargo, M.D., professor of Genomic Medicine and Surgical Oncology. “Our study sheds light on the potential effects of a patient’s diet and supplement use when starting treatment with immune checkpoint blockade. These results provide further support for clinical trials to modulate the microbiome with the goal of improving cancer outcomes using dietary and other strategies.”
Patients who reported eating more fruits, vegetables, legumes and whole grains met the study threshold for sufficient fiber intake. The 37 patients with sufficient fiber intake had improved progression-free survival (median not reached) compared to the 91 patients with insufficient fiber intake (median 13 months). Every five-gram increase of daily fiber intake was associated with a 30% lower risk of cancer progression or death.
When the patients were further grouped according to high- or low-fiber diet and commercially-available probiotic supplement use, response to immunotherapy was seen in 18 of 22 patients (82%) who reported both sufficient fiber intake and no probiotic use, compared to the response seen in 60 of 101 (59%) patients who either reported insufficient fiber intake or probiotic use. Response was defined as complete or partial complete or partial tumor shrinkage or stable disease for at least six months. Probiotic use alone was not associated with a significant difference in progression-free survival or odds of response to immunotherapy.
“Dietary fiber is important for gut health, just as it's important for overall health, and the two things are very tightly intertwined,” said co-senior author Carrie Daniel-MacDougall, Ph.D., associate professor of Epidemiology. “In this study, we saw that dietary fiber also may be important to cancer treatment, which brings us to a point where we can design interventional studies to answer the questions that patients really want answered: ‘Does what I eat now matter and could it impact my treatment outcome?’ We’re united in working to find answers for our patients.”
Differences in gut microbiota and pre-clinical models
The study began with analyzing the gut microbiome profiles of 438 melanoma patients, 321 of whom had late-stage disease and were treated with systemic therapy, and 293 of whom had an evaluable response to treatment over follow-up. The majority of these patients (87%) received immune checkpoint blockade, most commonly PD-1 inhibitors. A total of 158 patients also completed a lifestyle survey of antibiotics and probiotics usage; of these, 128 completed a dietary questionnaire as they began immune checkpoint therapy.
The research team reinforced their prior findings, which showed a higher abundance of Ruminococcaceae and Faecalibacterium prausnitzii – well-known and potentially beneficial bacteria involved in the digestion of fiber or starch – in patients who responded to immunotherapy. In contrast to the previous findings, overall diversity of gut bacteria was not associated with response to immunotherapy, potentially due to the larger size of this patient cohort.
The researchers also tested higher versus lower fiber diets and probiotic use in several preclinical melanoma models to shed light on the potential mechanisms behind the observational findings from the patient cohorts. In multiple models, probiotic use was associated with impaired response to immune checkpoint blockade, larger tumors, lower gut microbiome diversity and less cytotoxic T cells in the tumor microenvironment. A high-fiber diet was associated with slower tumor growth and significantly higher frequency of CD4+ T cells in pre-clinical models treated with PD-1 inhibitors.
Clinical trial to build on findings, test effect of dietary intervention
Based on the early study findings, a randomized clinical trial (NCT04645680), led by co-first author Jennifer McQuade, M.D., assistant professor of Melanoma Medical Oncology, will examine how whole-food-based diets with varying fiber content affect the microbiome and immune response. The study is currently enrolling patients with stage III-IV melanoma who are receiving immunotherapy.
“Our research teams within the Program for Innovative Microbiome and Translational Research (PRIME-TR) and Bionutrition Research Core at MD Anderson are collectively working to transform cancer therapy by modifying the microbiome,” Wargo said. “We’re grateful to the patients and families who have participated in our research and are hopeful that this work will ultimately provide evidence-based guidance to help patients take control of their own diets to improve their odds against cancer.”
The study was supported by the Melanoma Moon Shot®, part of MD Anderson’s Moon Shots Program®, a collaborative effort designed to accelerate the development of scientific discoveries into clinical advances that save patients’ lives. Additional research support included funding from National Institutes of Health and National Cancer Institute (1R01 CA219896-01A1, P30 CA016672), the Andrew Sabin Family Fellowship Program, Department of Defense, MD Anderson Multidisciplinary Research Program, MD Anderson Physician Scientist Program, Melanoma Research Alliance, Sean Parker Institute for Cancer Immunotherapy at MD Anderson, Stand Up to Cancer and U.S.-Israel Binational Science Foundation.
In addition to Wargo and Daniel-MacDougall, MD Anderson co-senior authorship also included Lorenzo Cohen, Ph.D., professor of Palliative, Rehabilitation and Integrative Medicine. Wargo is an inventor on a U.S. patent application that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome. A full list of co-authors and their disclosures can be found in the paper.

Metastatic melanoma survivor grateful for immunotherapy clinical trial
Last updated March 18, 2022.
When Bob Seibert reflects on the nearly one and half years since his stage IV melanoma diagnosis, there are several moments that he sees as divine intervention:
- First, a routine arterial plaque scan to check on his heart incidentally found a tumor in his lung – before he had any symptoms and “before it could have been much worse,” Bob says.
- Second, his wife happened to run into an acquaintance who recommended MD Anderson the same day he received his initial PET scan results.
- Third, the immunotherapy combination he received in a clinical trial was approved by Food and Drug Administration (FDA) and is now the new standard of care for people with advanced melanoma.
Since Bob enrolled in the clinical trial in January 2021, his tumors have all shrunk significantly or completely disappeared. “I feel so blessed and humbled with the way things have worked out,” he says.
The Phase I clinical trial tested the combination of nivolumab, an immune checkpoint inhibitor, and relatlimab, a new form of immunotherapy called a LAG-3 antibody, in two different doses. The dose Bob received was used in the Phase II clinical trial that led to the approval of the combination, marking the first time a LAG-3 antibody was approved for cancer treatment.
“We are incredibly excited to have a new standard of care to offer patients with advanced melanoma,” said Hussein Tawbi, M.D., Ph.D., who led the Phase I and III clinical trials at MD Anderson. “The approval of relatlimab represents a major, long-awaited step forward in immunotherapy treatment, and we’re grateful to all of the patients whose clinical trial participation made this advance possible.”
Immunotherapy provides hope for advanced melanoma
Bob knows that if he was diagnosed a decade earlier his story could have gone much differently. His primary care doctor had ordered the scan as a baseline test, and they were both surprised when it picked up a large tumor in his lungs. After a biopsy, Bob learned it was metastatic melanoma.
“Both my local oncologist and Dr. Tawbi at MD Anderson said if I was seeing them 10 years ago, the message would have been to get my affairs in order because I wouldn’t have long left, but that immunotherapy had changed the game,” Bob says. “They said I could expect to have at least two good years – and possibly longer, thanks to immunotherapy.”
Immunotherapy has revolutionized cancer treatment, beginning with the approval of CTLA-4 inhibitor ipilimumab for advanced melanoma in 2011. MD Anderson immunologist Jim Allison, Ph.D., was awarded the 2018 Nobel Prize in Physiology or Medicine for his work that led to development of the drug. Other immunotherapies have followed since then, including PD-1 inhibitors nivolumab and pembrolizumab.
“Ever since we proved that ipilimumab works, the goal has been to make immunotherapy work better for more people,” Allison says. “It’s incredibly gratifying to see the pace of progress to find a third immune checkpoint pathway, and I fully expect to see even more meaningful advances in immunotherapy for patients and their loved ones in the future.”
Choosing a clinical trial for the newest immunotherapy combination
Bob’s oncologist in South Carolina gave him two options: single-agent immunotherapy with pembrolizumab or combination immunotherapy with ipilimumab and nivolumab. The combination therapy was more effective, but it also had a greater chance of significant side effects, and Bob’s melanoma wasn’t quite extensive enough for the doctor to recommend starting with the more aggressive therapy.
Bob had already decided to get a second opinion from a specialized cancer center. He made an appointment at MD Anderson, after some research and advice from friends. Here, he met with Tawbi, who agreed with what Bob had already heard about the standard treatment options. He also presented Bob with a third choice: sign up for a clinical trial.
Tawbi detailed two different clinical trials that were testing the combination of nivolumab and relatlimab.
“When Dr. Tawbi explained that relatlimab and nivolumab appeared to be just as effective as ipilimumab and nivolumab, but with almost zero additional toxicity compared to nivolumab by itself, I said, ‘Sign me up!’” Bob recalls. “It seemed so obvious that’s what I needed to do.”
Soon after he met Tawbi, Bob had a colonoscopy, which revealed another metastatic melanoma tumor. At that point, he was even more relieved to have his combination immunotherapy treatment plan already in place.
Shrinking tumors without side effects
Bob had his first infusion of relatlimab and nivolumab in January 2021, and he has continued to receive the immunotherapy combination every month since then. His first post-treatment scans in March 2021 showed no tumor growth. Some of his tumors had already disappeared, while others shrank to half their original size or less. Bob had been careful not to set his expectations too high and was surprised by how quickly the treatment worked against his cancer.
“It was pretty powerful news,” Bob says. “Dr. Tawbi came in the room, high-fived me and said it was as good of an initial response as he’s seen. So many people had been praying and rooting for me. It was very humbling and gratifying.”
His tumors have continued to shrink and now measure less than 2 cm total. On top of that, his side effects have been minimal. He’s had a rash that comes and goes, minor joint pain, and fatigue that has shortened – but not prevented – his beloved fishing trips. In the airport on a recent visit to Houston, Bob had a rare day of feeling sick to his stomach and lousy enough that he considered not getting on the plane.
“Then it dawned on me: if I was on any form of chemo or radiation, how I feel right now would probably be wonderful in comparison,” he says. “I don’t ask myself, ‘Why me?’ about having melanoma, but about why I’ve had it so easy and my recovery been so complete. I see God’s grace in the work that’s done at MD Anderson and has seemed to heal me so far.”
Request an appointment at MD Anderson online or by calling 1-877-632-6789.
