Research
Apoptosome Model
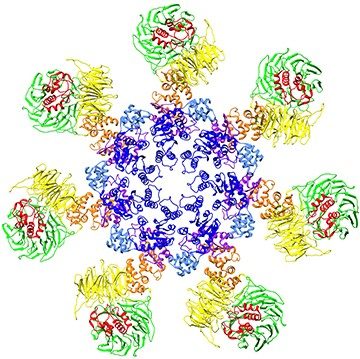
Caspase-activating Complexes
Most toxicants induce apoptosis by triggering the activation of caspases (cysteinyl aspartate-specific proteases). Initiator caspases are activated through their association with specific adapter proteins, and in turn, activate effector caspases that cleave cellular proteins and dismantle the cell. Thus, caspases are activated through unique signal transduction pathways. The overarching goal of research in the Bratton lab is to understand how caspases are activated and how their activities are modulated. The lab is particularly interested in the apoptosome. This large multimeric complex is composed of seven Apaf-1 (apoptosis protease-activating factor-1) proteins and is formed in response to mitochondrial stress. It binds to the initiator caspase-9 to form a holoenzyme that subsequently activates the effector caspases-3 and -7. The Bratton group is currently defining the regulatory mechanisms that control the activity of this complex both in vitro and in vivo.

Inhibitor of Apoptosis (IAP) Proteins and IAP Antagonists
IAPs are important antiapoptotic proteins present throughout nature. In humans and rodents, at least one family member, XIAP (X-linked IAP), is an established inhibitor of caspases-9, -3 and -7. However, it remains unclear how other family members suppress apoptosis. The Bratton lab is investigating how other IAPs, including cellular IAP1 (cIAP1) and cIAP2, and Drosophila IAP-1 (DIAP1) and DIAP2, suppress apoptosis in human and fly models of cell death. The fly IAP antagonists, Reaper, Hid (head involution defective), Grim, Sickle, and dOmi, have been thought to induce apoptosis during development by antagonizing DIAP1. However, recent studies from both the Bratton laboratory and other groups indicate that these IAP antagonists may promote cell death through mechanisms that do not involve antagonism of DIAP1 per se. Thus, Bratton lab researchers are actively seeking to define these alternative pathways to cell death.
Heat Shock-induced Apoptosis
Hyperthermia (heat shock therapy) is being tested in phase II/III clinical trials (both alone and in combination with radiation or chemotherapy), as a cancer treatment. Unfortunately, though intense heat shock induces apoptosis, the underlying mechanisms remain controversial and unclear. The lab has discovered that heat shock induces caspase-dependent apoptosis through mechanisms that do not require any of the known initiator caspases or their activating complexes. The group is now working to identify the mechanisms responsible for heat shock-induced apoptosis, including the identification and characterization of the apical protease(s) responsible for initiating the caspase cascade.
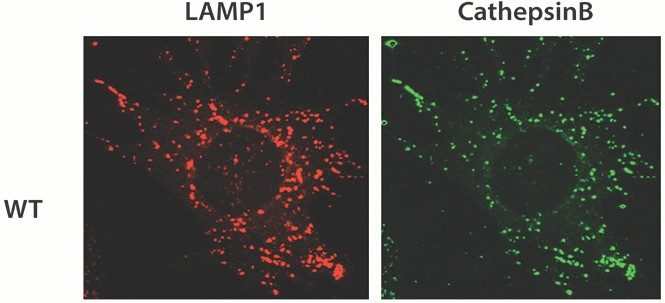
Heat shock can induce apoptosis. The Bratton lab has hypothesized that heat shock induces cell death, in part by stimulating rapid endo-lysosomal membrane permeabilization (ELMP), which coincides with cytosolic acidification and release of cathepsins into the cytoplasm. In the image to the left, they used LAMP1 as a marker of lysosomal membranes, and imaged the release of cathepsin B, a cysteine protease stored in the lysosome, to study the effects of heat shock.
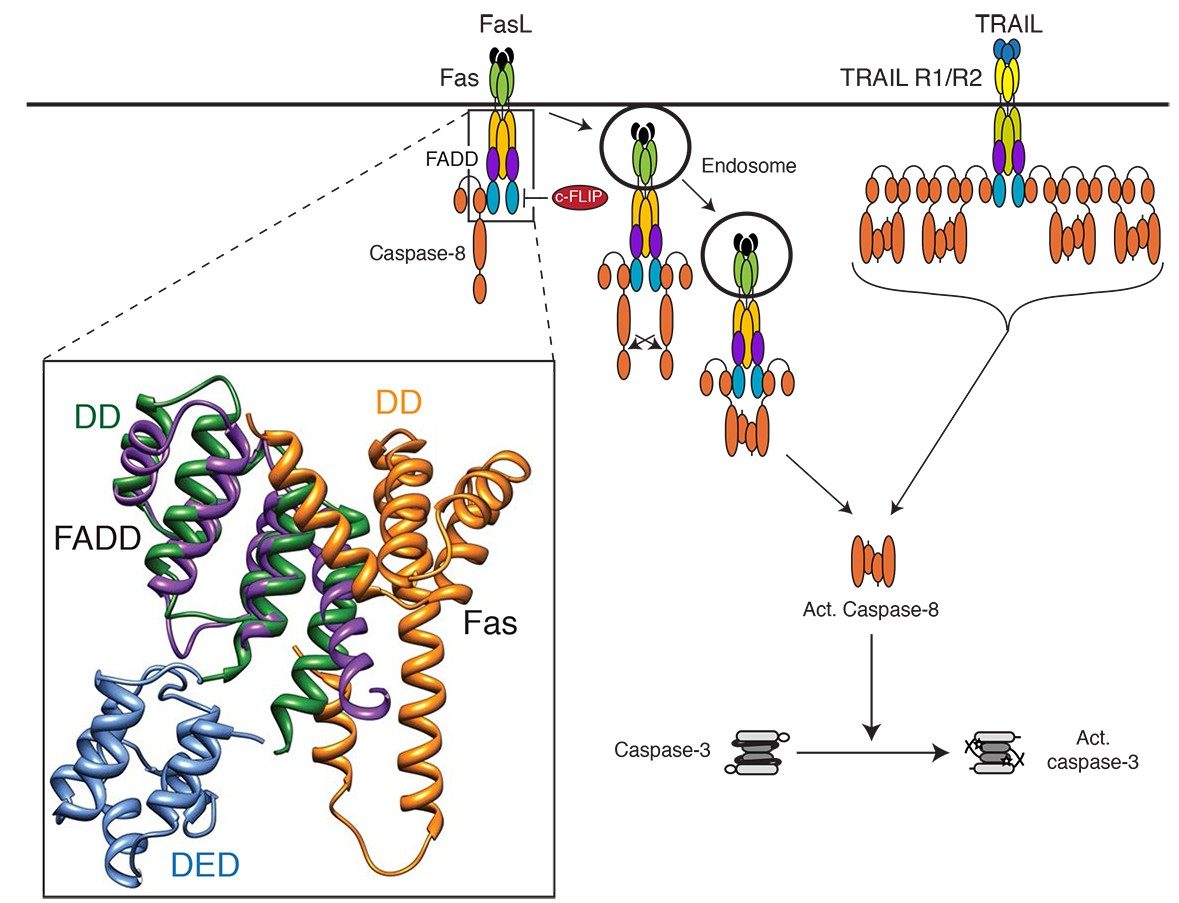
TRAIL-induced Apoptosis and Resistance in Prostate Cancer
Tumor cells are normally removed from the body, in part, through the activation of death receptors located on tumor cells, such as Fas/CD95 or death receptors-4 and 5 (DR4/5). These receptors are activated by their cognate ligands, FasL and Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), respectively. TRAIL can selectively induce apoptosis in cancer cells, and consequently has potential therapeutic value. Clinical trials are underway to test recombinant TRAIL and agonistic antibodies to DR4/5 as a cancer treatment. Unfortunately, ~50% of tumors exhibit resistance to TRAIL; therefore, the group is investigating the primary mechanisms of TRAIL resistance in prostate cancer, using both in vitro and animal models.
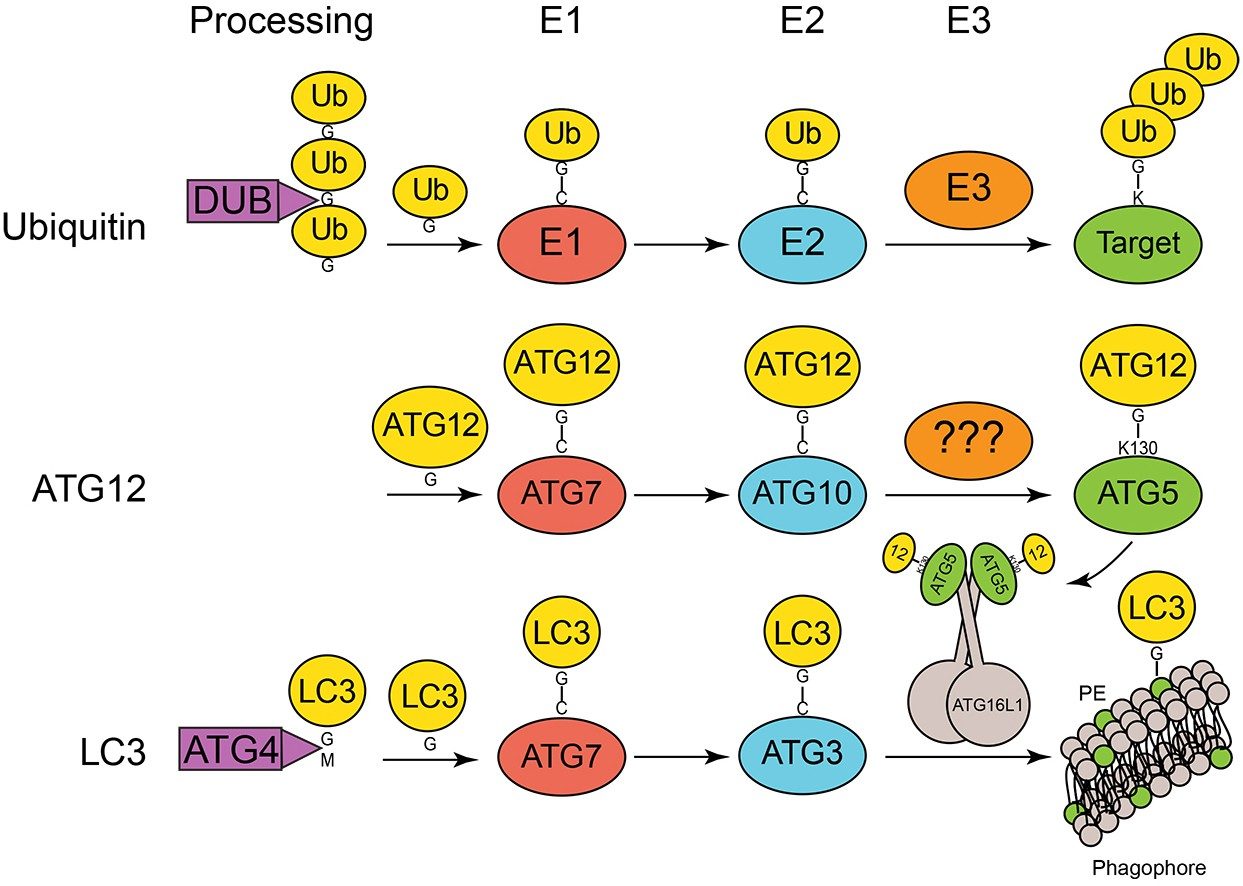
Assembly and Formation of the Autophagosome
Lysosomes and proteasomes are two primary sites for degradation within the cell. Macroautophagy, more simply called autophagy, involves the formation of double-membrane vesicles, called ‘autophagosomes,' that envelop cytoplasmic material and fuse with lysosomes. Upon fusion, the sequestered material is degraded by lysosomal proteases. Autophagosome formation requires several autophagy-related (ATG) proteins, including ATG12, ATG5, and ATG16L1, which form a complex to catalyze the ubiquitin-like conjugation of LC3 directly to the expanding membrane of the 'phagophore,' which is the autophagosome precursor. Formation of this complex requires another ubiquitin-like conjugation reaction that covalently links ATG12 to ATG5. The Bratton group is defining both the mechanisms regulating these essential ubiquitin-like conjugation reactions and the mechanisms guiding ATG12–ATG5-ATG16L1 complex assembly and disassembly.
The Role of ATG Genes and Autophagy in Prostate Cancer
The ubiquitin-proteasome system normally targets only short-lived, soluble proteins. In contrast, autophagy can target damaged or excess organelles, large protein aggregates, and invading pathogens both to maintain cell homeostasis and to facilitate cell survival in response to nutrient, oxidative, chemotherapeutic and other cellular stresses. Consequently, autophagy plays a complex and contextually specific role in tumorigenesis, tumor progression, and metastasis that is incompletely understood. The Bratton lab is working to decipher how autophagy is regulated and to define the specific functions of ATG genes and autophagy in human and murine prostate cancers.
