Research
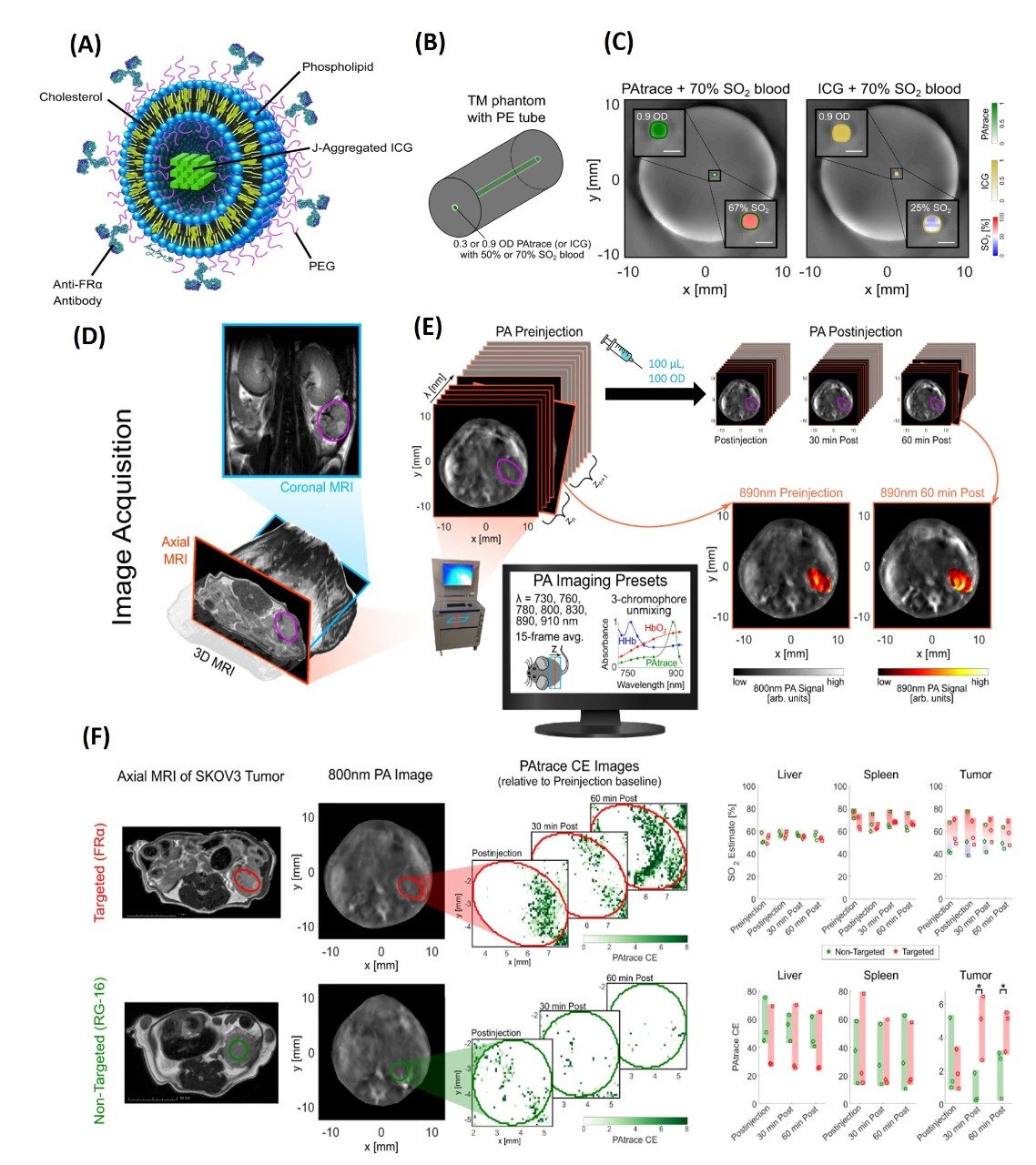
Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregate
Molecular imaging is becoming a key tool in both diagnostics and therapy with the emergence of patient-specific treatment schemes to allow for real-time visualization of chemical and biological processes. Of the clinically viable molecular imaging strategies being explored preclinically, photoacoustic (PA) imaging is one of the most promising, making inroads into the clinic due to its nonionizing nature and ability to resolve optical contrast at clinically relevant depths with high spatiotemporal resolution. Widespread use of PA imaging, however, is limited by currently available contrast agents, which either lack PA-signal-generation ability for deep imaging or their absorbance spectra overlap with hemoglobin, reducing sensitivity. Ideally, clinically translatable PA contrast agents should provide high contrast at depth after spectral unmixing from background hemoglobin, maintain molecular specificity to cellular targets, afford sufficient stability during imaging, and have a composition amenable to translation. One dye in particular that has gained significant attention for PA imaging due to its FDA approval status is indocyanine green (ICG). In addition, liposomes have been extensively researched as nanocarriers for drug delivery as they allow for targeted delivery of chemotherapeutics while reducing toxicity to normal organs, with a few such formulations having already made it into the clinic.
This project was started with the goal of developing a robust PA contrast agent based on particular specifications that need to be achieved for a successful clinical translation, including (i) high absorbance in the NIR optical tissue window; (ii) the possibility of quantitative combined functional (e.g., SO2) and molecular PA imaging; (iii) stability during storage and in vivo applications; (iv) molecular specificity; and (v) reduction of regulatory approval burden. In order to address the aforementioned limitations, we report on a PA contrast agent based on targeted liposomes loaded with J-aggregated indocyanine green (ICG) dye (i.e., PAtrace) that we synthesized, bioconjugated, and characterized. Our agent, PAtrace, was built around ICG dye because of its optical absorbance in the NIR and its FDA approval status. Then, PAtrace’s absorbance and PA signal generation were significantly increased by forming J-aggregates that allowed sensitive detection at clinically relevant depths. Further, shifting the absorbance peak to ~890 nm eliminated overlap with distinctive spectral features of hemoglobin that are commonly used for blood oxygenation measurements. Our study results show that PAtrace concurrently provides significantly improved contrast-agent quantification/sensitivity and SO2 estimation accuracy compared to monomeric ICG. Overall, PAtrace’s performance attributes and composition of FDA-approved components make it a promising agent for future clinical molecular PA imaging.
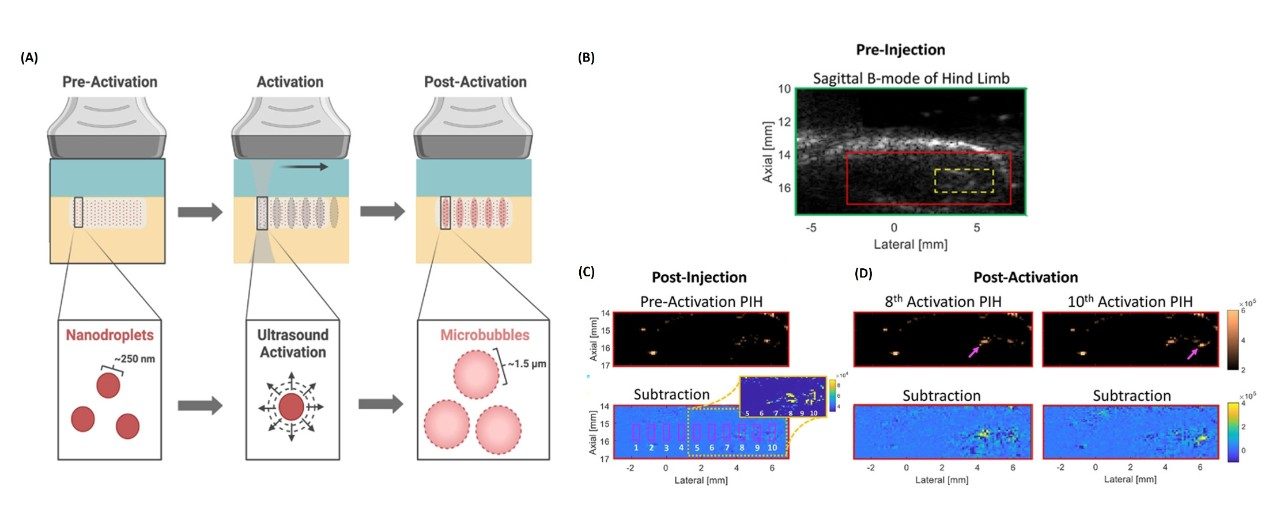
Effect of perfluorocarbon composition on activation of phase-changing ultrasound contrast agents
Microbubble contrast agents (MCAs) can be used to achieve enhanced ultrasound (US) contrast due to their gaseous core and micron-order size, however, their size does not allow for vascular extravasation (e.g., via the enhanced permeability and retention effect), which limits them to intravascular delivery. Alternatively, nanoscale agents can be used for extravascular delivery. This discrepancy in optimal agent properties for vascular extravasation and US imaging has led to the development of perfluorocarbon (PFC)-cored phase-changing nanodroplet contrast agents (PNCAs). Phase-changing nanodroplet contrast agents (PNCAs) can be extravascularly delivered, and focused US pulses can be used to locally “activate” them so they can convert to MCAs and be visualized with high contrast using nonlinear US. Although therapeutic US activation sequences laser activation of optically loaded PNCAs with and without acoustic activation have proven successful despite potential safety and penetration-depth limitations, there has been minimal success to date in the activation of C6-core PNCAs with diagnostic US due to their relatively high activation threshold (aT). Due to the higher aT inherent to these stable particles, higher-pressure transmits are necessary for their activation, thereby increasing the risk of producing harmful bioeffects (e.g., hemolysis and capillary endothelial injury).
The goal of this project involves investigating the effect of perfluorocarbon (PFC) core composition and presence of cholesterol in particle coatings on stability and image contrast generated from acoustic activation of PNCAs using high-frequency US suitable for clinical imaging. Our most recent study approach characterized PCNAs with varied core compositions (i.e., mixtures of perfluoropentane [C5] and/or perfluorohexane [C6]) and two coating formulations (i.e., with and without cholesterol) in order to investigate thermal/temporal stability and postactivation, nonlinear US contrast in phantom and in vivo environments. This preliminary data shows that C6-core PNCAs can provide high-contrast US imaging with minimal nonspecific activation in phantom and in vivo environments. Future work will explore the optimization of PNCA coatings to ensure that samples are readily activatable with minimal spontaneous activation for continued in vivo translation. Additionally, PNCAs with functionalized lipids will be conjugated to antibodies to investigate targeting efficacy.
