Research

Our laboratory focuses on three areas to improve care for patients with ovarian cancer and other malignancies: early detection, overcoming drug resistance, and targeting tumor dormancy and autophagy.

Ovarian Cancer Early Detection
Identification of biomarkers and strategies for early detection of ovarian cancer
Ovarian cancer is a leading cause of cancer death from gynecologic malignancies that is diagnosed each year in more than 300,000 women worldwide and 19,880 from the United States alone. Despite advances in treatment, 70-75% of women with ovarian cancer are diagnosed with advanced stage (III or IV) where the cure rate is less than 30%. At present, only 25-30 % of patients are currently diagnosed in early, potentially curable, stage I or II. Computer models suggest that mortality could be reduced by 10-30% if more ovarian cancers were detected at an earlier stage. Having developed the first monoclonal antibodies that react with epithelial ovarian cancers (JCI, 1981), our group discovered the CA125 (MUC16) glycoprotein, leading to the development of the first clinically useful biomarker for monitoring ovarian cancer that has been used worldwide in the care of hundreds of thousands of women (NEJM, 1983). Over the last 21 years, our team has conducted the NROSS trial using rising CA125 to trigger ultrasound and abnormal ultrasound to trigger surgery, detecting 74% of ovarian cancers with 70% in the early stage (I-II) (JCO, 2024). To improve this strategy, we have identified three additional blood biomarkers that detect 13% of cases missed by CA125 (Brit J Cancer, 2024). We are conducting the NROSS2 trial to test this 4-biomarker panel. Our lab continues to search for additional biomarkers to diagnose ovarian cancer even earlier, to detect minimal residual disease after primary treatment with surgery and chemotherapy, and to find disease recurrence sooner than with CA125. Autoantibodies can be induced by very small amounts of cancer. Anti--TP53 autoantibodies detect >20% of ovarian cancers 8 months before CA125 or 22 months before diagnosis when CA125 is not elevated (Clin Cancer Res, 2017). A panel of autoantibodies is being developed to provide earlier detection than with our current four-biomarker panel. We are also collaborating in testing proteomic and metabolomic biomarkers that might improve upon currently available tests.

Funding Support
NCI EDRN (U01 CA200462; Bast, PI)
(R01 CA247220; Lokshin and Bast, PI’s)
Drug Resistance
Individualized enhancement of primary sensitivity to paclitaxel in ovarian cancer
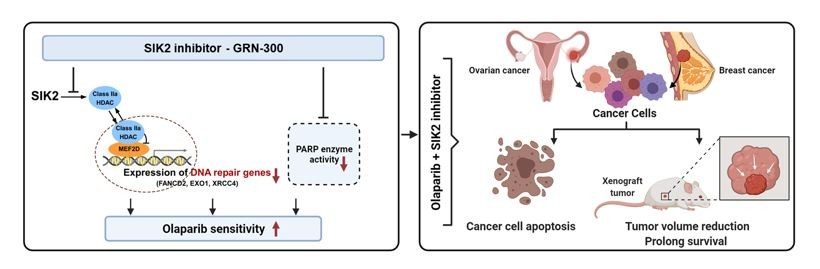
Most ovarian cancer patients receive a combination of carboplatin and paclitaxel chemotherapy, but less than half of their cancers respond to paclitaxel. Enhanced response to primary chemotherapy could improve outcomes for the majority of non-responders by combining targeted therapy with paclitaxel. Using a kinome siRNA screen, we identified more than 30 kinases that regulate paclitaxel sensitivity in ovarian cancer cells (Cancer Res, 2011). Knockdown of kinases such as IKBKB and STK39 enhances paclitaxel sensitivity by increasing microtubule stability regulated by microtubule-associated protein 4 (MAP4; Clin Cancer Res, 2020). Knockdown of kinases that modulate the pentose phosphate shunt such as PFKFB2 also enhances the response to paclitaxel in ovarian and breast cancer cells with wild-type TP53 (Clin Cancer Res, 2019). Knockdown of Salt-inducible Kinase 2 (SIK2) enhanced sensitivity to paclitaxel, inducing polyploidy and inhibiting PI3K activity. SIK2 encodes an AMP-like kinase that is overexpressed in 30% of ovarian cancers, associated with decreased survival. We discovered that SIK2 localizes to the centrosome and is required for centrosome splitting (Cancer Cell, 2010). It also regulates PI3 kinase activity and class Iia HDAC phosphorylation and nuclear localization (JCI, 2022). SIK2 has been inhibited with the low molecular weight inhibitors ARN-3236 and GRN-300. GRN-300 sensitizes ovarian cancer cells to paclitaxel, carboplatin, and PARP inhibitors by different mechanisms. GRN-300 also enhances immune checkpoint therapy with anti-PD-L1 in murine models by enhancing T-cell recruitment. A Phase I clinical trial of a SIK2 inhibitor (GRN-300) with paclitaxel is underway with little toxicity.
Funding Support
Ovarian SPORE (P50CA28170; Sood and Bast, PI’s)
(ACS SRA-22-193; Bast, PI)

Tumor Suppressor and Dormancy
DIRAS3 is an imprinted tumor suppressor gene that is down-regulated in ovarian cancers through genetic and epigenetic mechanisms
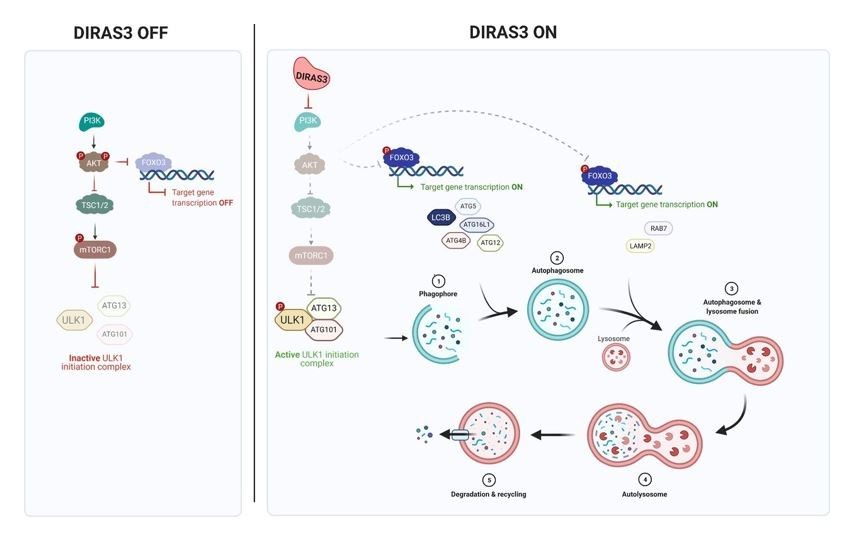
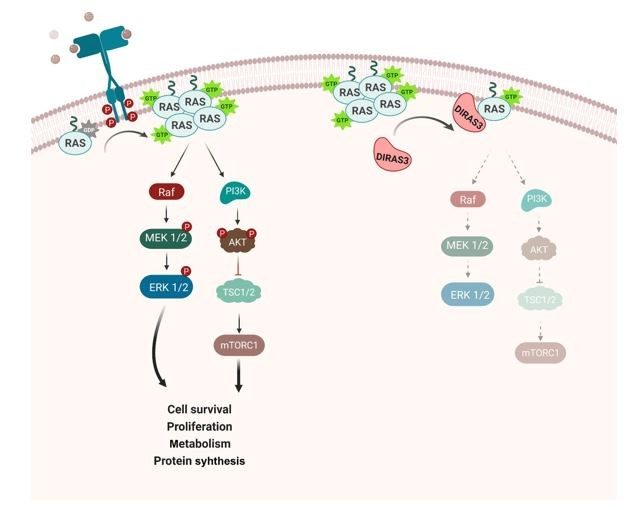
DIRAS3 (ARHI) is an imprinted tumor suppressor gene that encodes a 26 kDa GTPase with 60% amino acid homology to RAS but with a distinctive 34 amino acid N-terminal extension required to block RAS function. DIRAS3 is maternally imprinted and expressed only from the paternal allele in normal cells. Loss of expression can occur in a single "hit" through multiple mechanisms (Clin Cancer Res, 2005). Downregulation of DIRAS3 occurs in cancers of the ovary, breast, lung, prostate, colon, brain, and thyroid (PNAS, 1999). Re-expression of DIRAS3 inhibits signaling through PI3 kinase/AKT, JAK/STAT, and RAS/MAPK, blocking malignant transformation, inhibiting cancer cell growth and motility, and preventing angiogenesis (MCT, 2022). DIRAS3 is essential for autophagy and triggers this process through multiple mechanisms. Re-expression of DIRAS3 induces dormancy in a nu/nu mouse xenograft model of ovarian cancer, inhibiting cancer cell growth and angiogenesis (JCI, 2008). DIRAS3-mediated induction of autophagy facilitates the survival of dormant cancer cells in a nutrient-poor environment (Autophagy, 2014). DIRAS3 expression in dormant, drug-resistant autophagic cancer cells can serve as a biomarker and as a target for novel therapy to eliminate the residual disease that remains after conventional therapy. DIRAS3 is a unique endogenous RAS inhibitor that binds directly to RAS, disrupting RAS dimers and clusters, and preventing RAS-induced transformation (Cell Rep, 2019). DIRAS3-related stapled peptides enter cells, disrupt RAS signaling, inhibit tumor growth, and enhance response to autophagy inhibitors. Expression of DIRAS3 or treatment with DIRAS-related stapled peptides can sensitize RAS-driven low-grade serous and ovarian cancers to anti-autophagic therapy (Autophagy, 2024).
Funding Support
(R01CA266187; Bast and Lu, PI’s)